The 1st Major Advance in Spinal Fusion Bone Growth Stimulator Technology in Nearly 20 Years
The 1st Major Advance in Spinal Fusion Bone Growth Stimulator Technology in Nearly 20 Years


The ActaStim System
Intentionally designed to improve patient compliance and spinal fusion success

Continuous wearability supports all-day healing
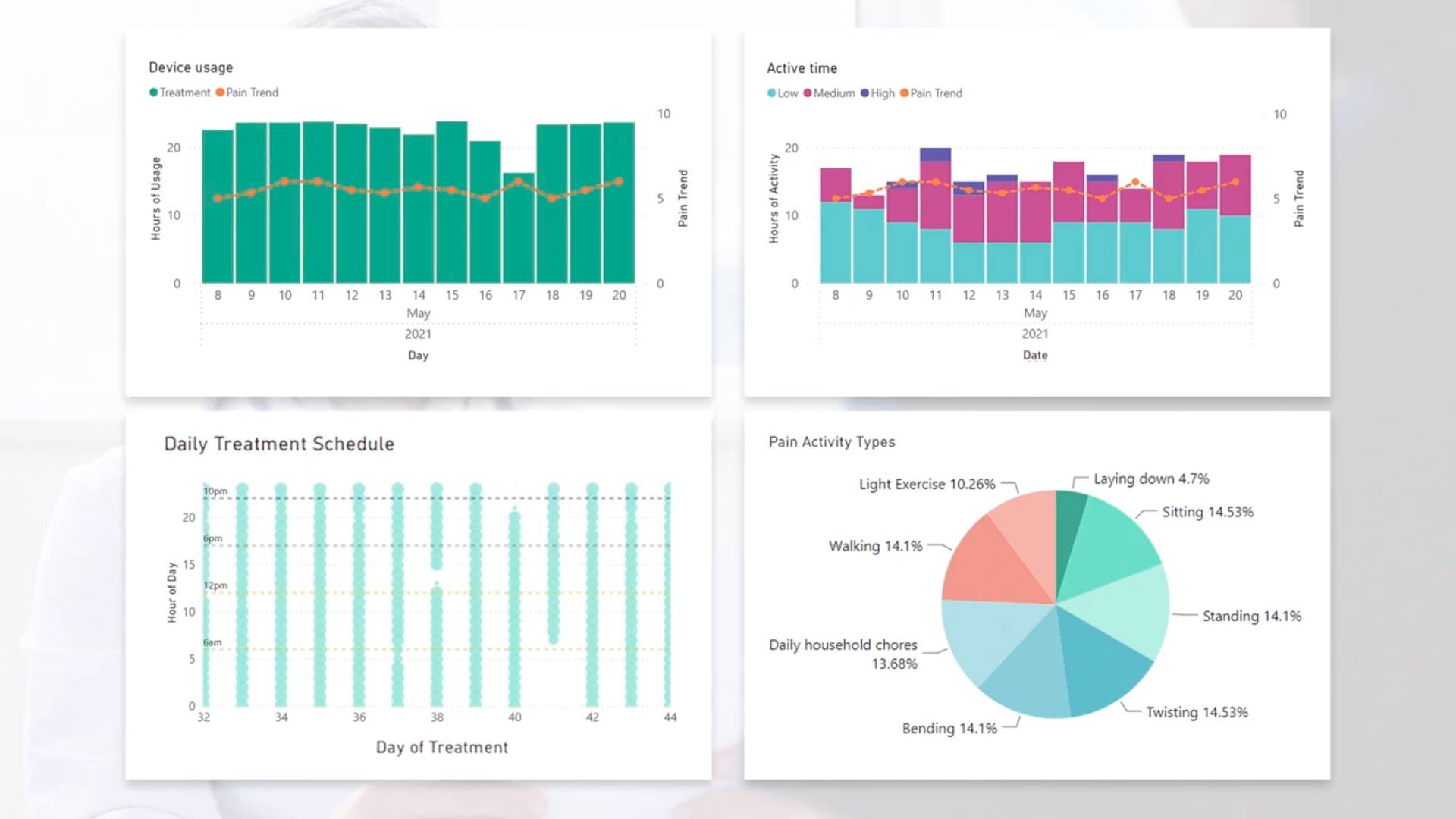
Daily insights to help guide recovery
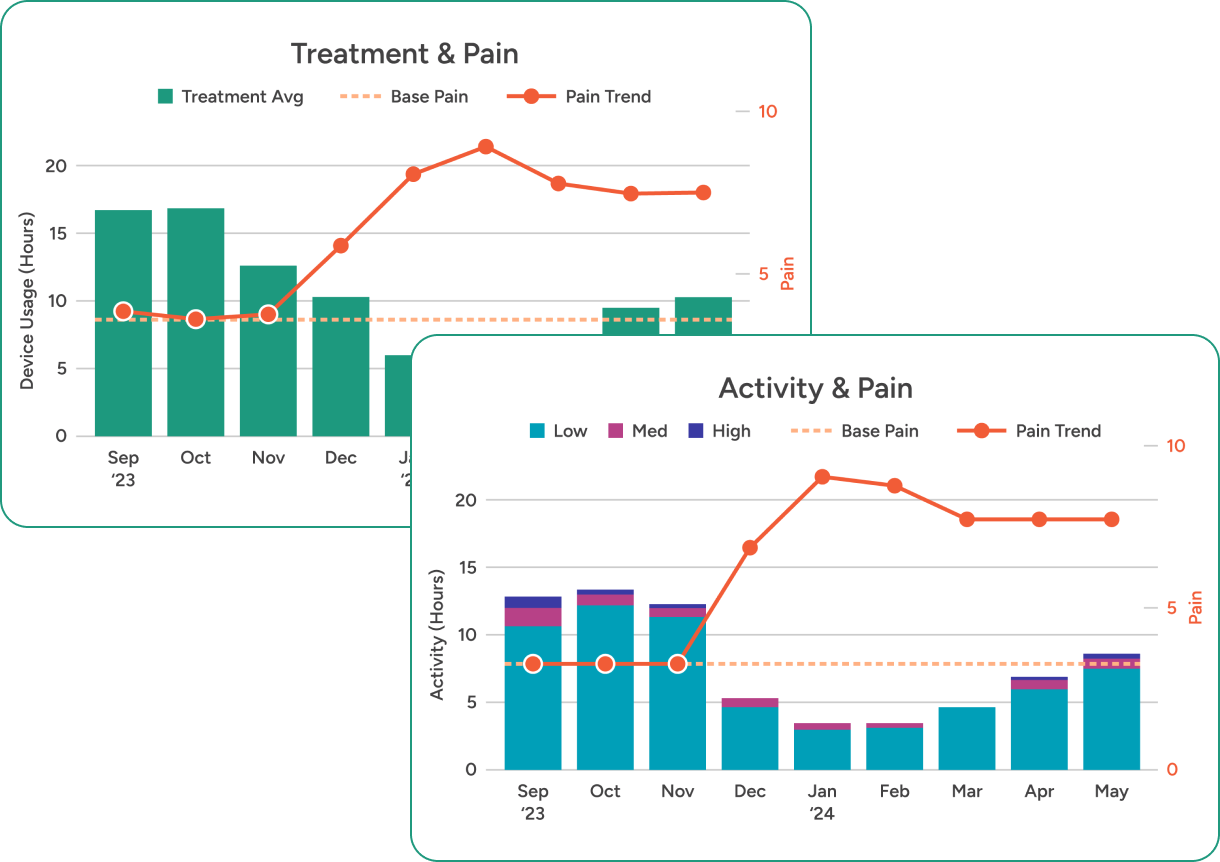
Clear charts and graphs combine auto-captured wear time & relative activity level data with self-reported pain scores. So it’s easy to see when patients get off track — and how to guide them back in the right direction.
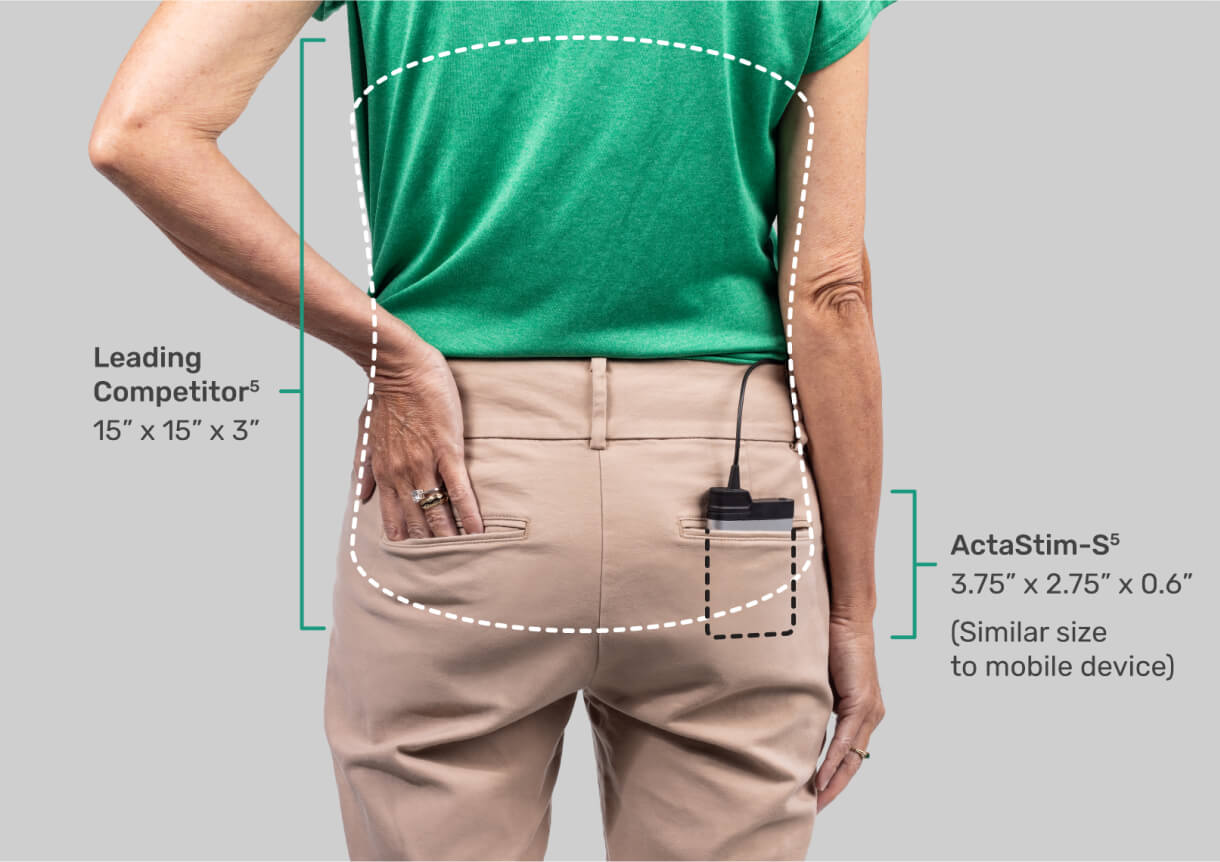
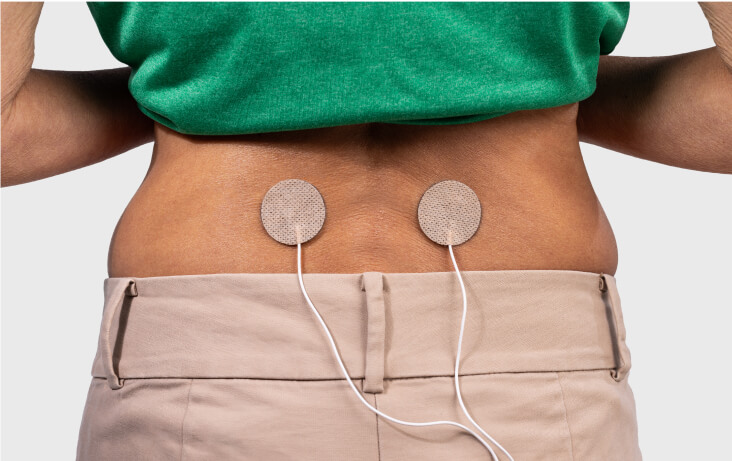
CONTINUOUS WEARABILITY
Fits into your patient’s pocket — and their life

CONTINUOUS WEARABILITY
Fits into your patient’s pocket — and their life
system COMPARISON
ActaStim vs. legacy spinal fusion bone growth stimulators
SAFE & EFFECTIVE
Double the chance for a successful outcome
increase in fusion success, regardless of the type of fusion2
as likely to have a successful outcome1
reduction in
failure rates2
reduction in failure rates2

You treat patients — not x-rays
Built on trusted technology

More potential healing benefits
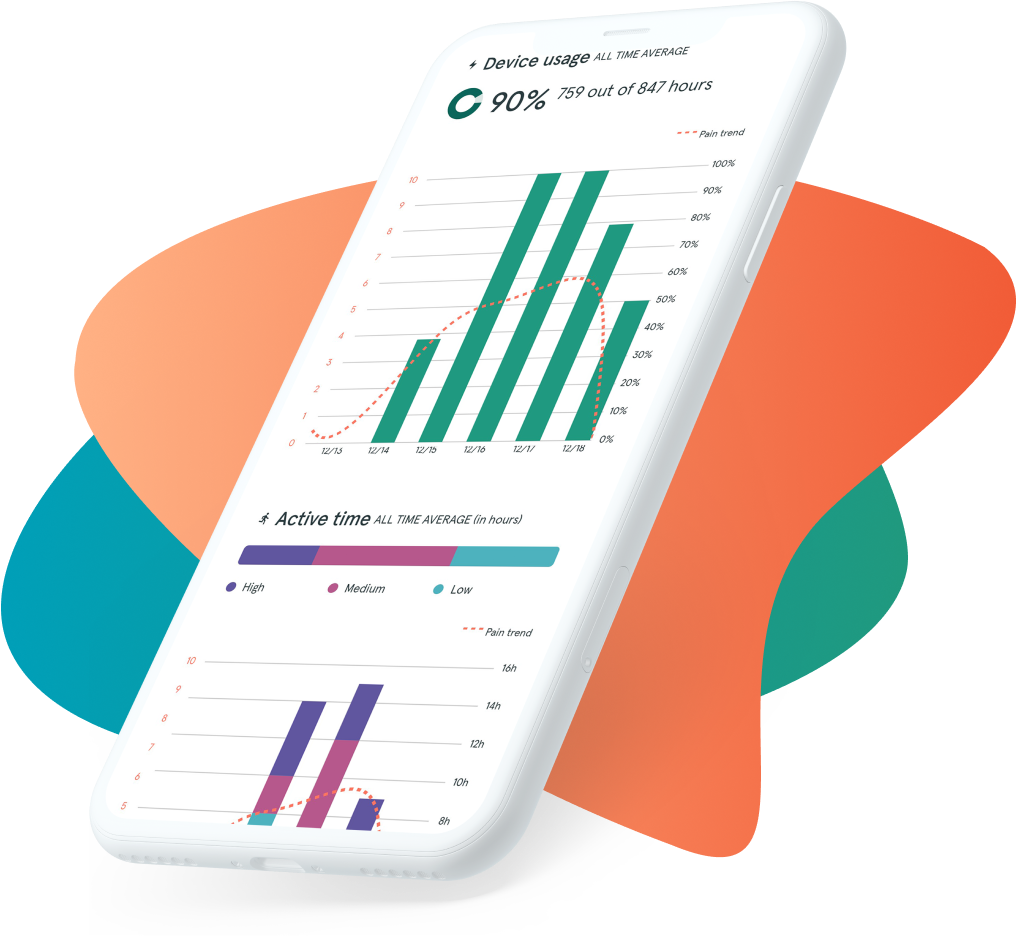
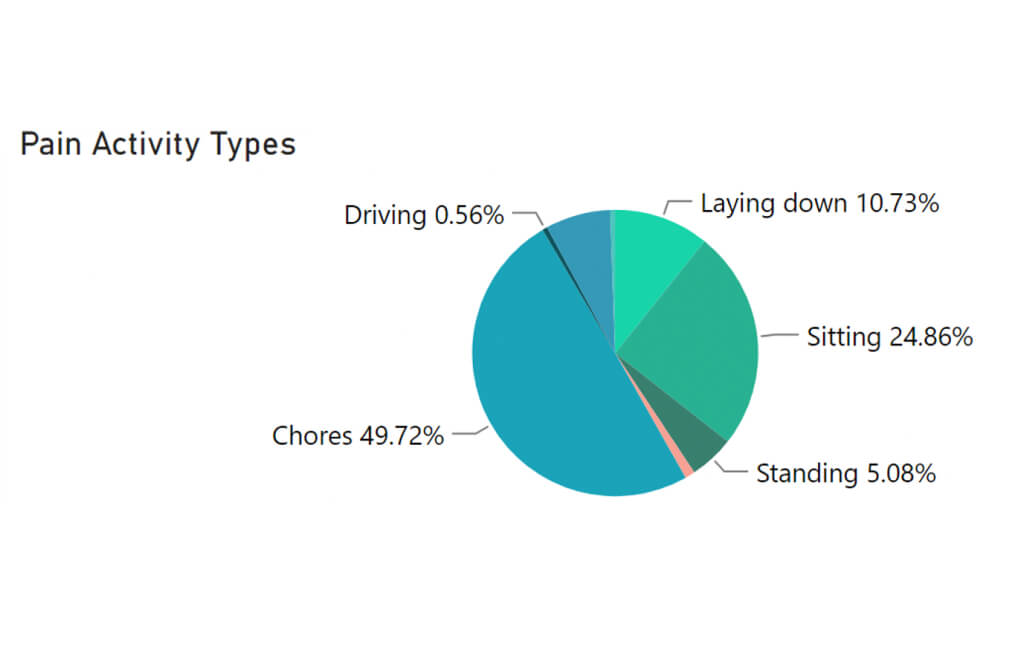
DAILY INSIGHTS
A new window into patient progress
- Automatically collects accurate, continuous recovery metrics
- Keeps patients engaged by letting them enter and manage data in the user-friendly ActaStim Sync app
- Combines all that data into reports that show you at-a-glance whether patients are staying on track
DAILY INSIGHTS
A new window into patient progress
True wear time
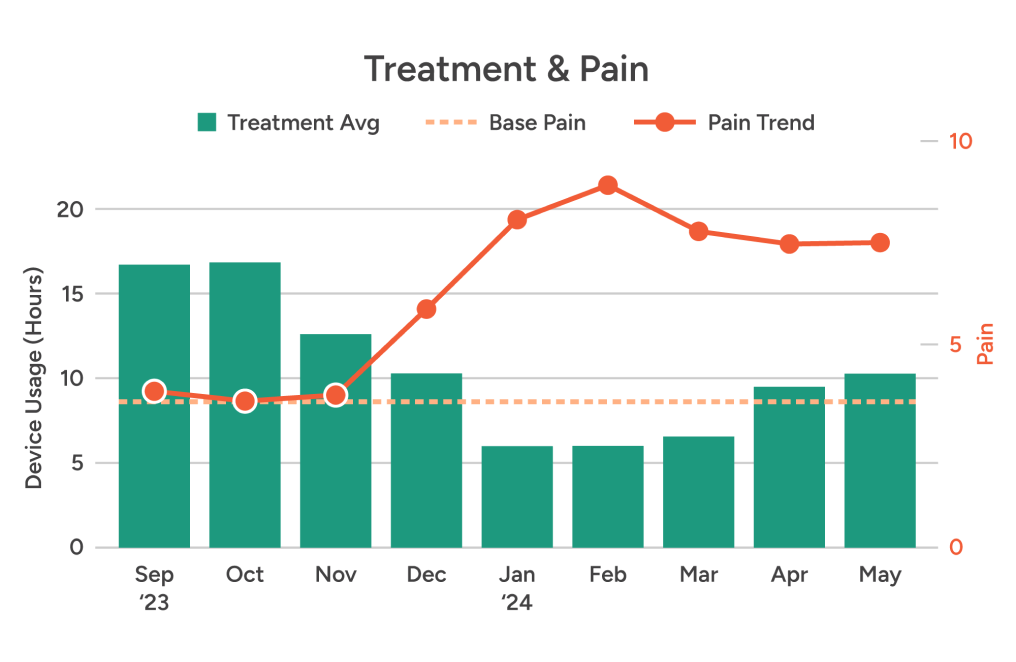
Relative physical activity
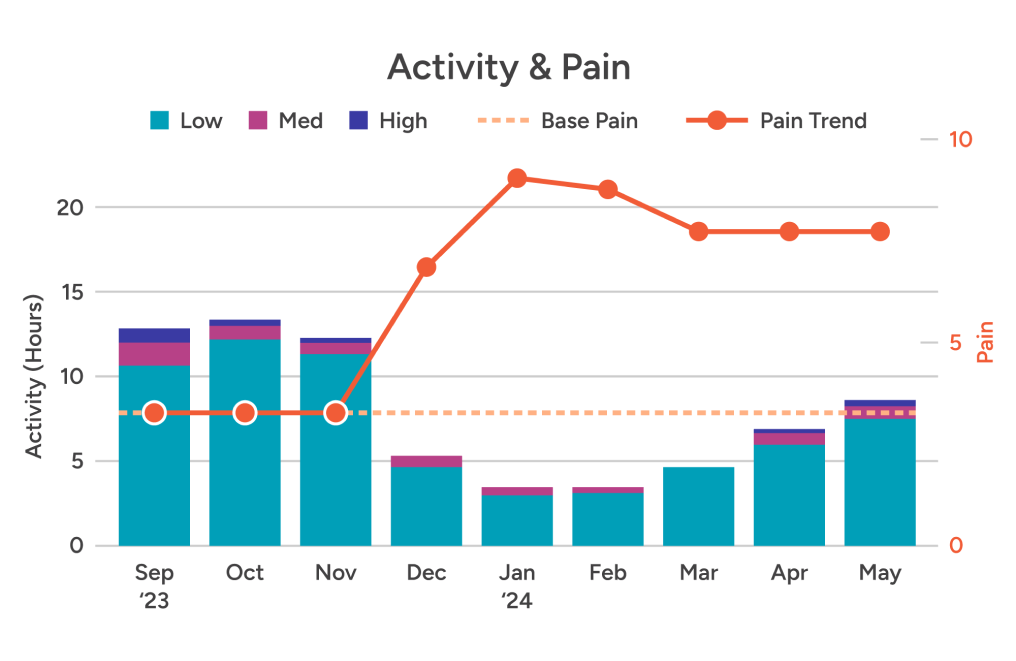
Patient-reported pain & more
VIP CUSTOMER CARE
For your patients & your practice

VIP CUSTOMER CARE
For your patients & your practice
- We support your staff, keeping you in the loop while implementing your vision for each patient’s recovery.
- A dedicated rep helps every patient set up their device, download and use the app, and get ongoing support.
- Plus, we navigate the insurance claims process, optimizing coverage, and tailoring payment plans.
RESOURCES
Product Documents
FAQs
What healthcare providers may want to know
See all the ways ActaStim can take you further
References: 1. U.S. Food and Drug Administration. (2020). PMA P190030: FDA Summary of Safety and Effectiveness Data. 2.Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine. 1999;24(13):1349-1356. 3. Brighton CT, Wang W, Seldes R, Zhang G, Pollack SR. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am. 2001;83-A(10):1514-1523. 4. Mooney V. A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine. (Phila Pa 1976). 1990 Jul;15(7):708-12. doi: 10.1097/00007632-199007000-00016. PMID: 2218718. 5. Data on File. 6. American Medical Electronics. (1986). PMA P850007: Physio-Stim Summary of Safety and Effectiveness Data. 7. OrthoLogic. (1999). PMA P910066: Spinalogic Bone Growth Stimulator Summary of Safety and Effectiveness.
ActaStim™ (spine fusion stimulator) or its use is covered by one or more of the following U.S. patents:11,395,919; 11,426,574;11,717,685;11,759,