0 %
increase in fusion success, regardless of the type of fusion1
0 x
more likely to have a successful outcome2
0 %
reduction in
failure rates1
reduction in failure rates2






increase in fusion success, regardless of the type of fusion1
more likely to have a successful outcome2
reduction in
failure rates1
reduction in failure rates2
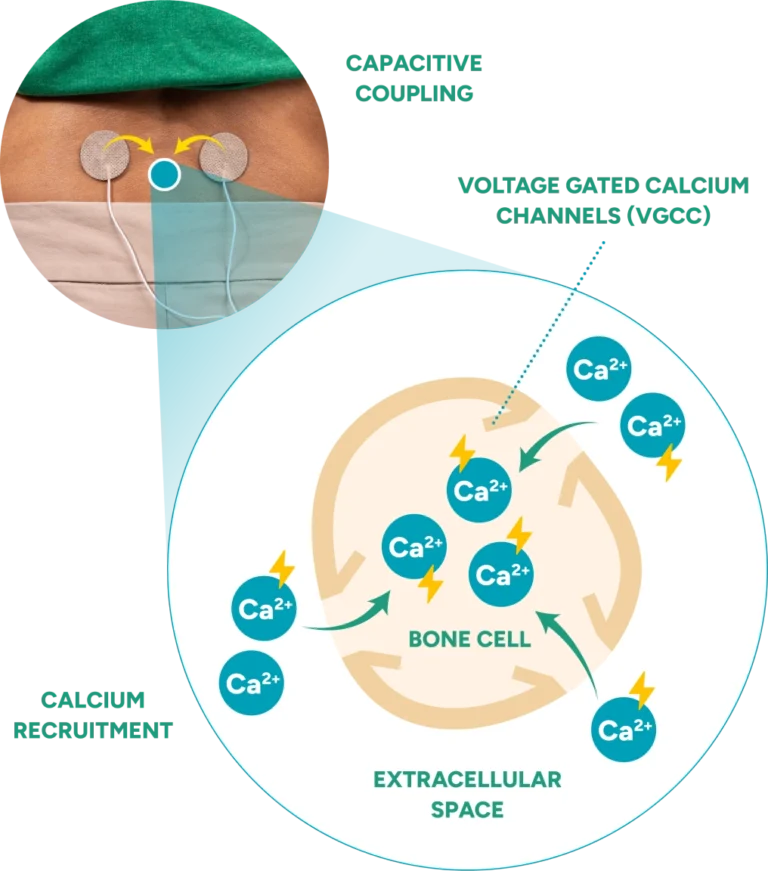

References: 1. U.S. Food and Drug Administration. (2020). PMA P190030: FDA Summary of Safety and Effectiveness Data. 2. Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine. 1999;24(13):1349-1356. 3. Brighton CT, Wang W, Seldes R, Zhang G, Pollack SR. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am. 2001;83-A(10):1514-1523. 4. Mooney V. A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine. (Phila Pa 1976). 1990 Jul;15(7):708-12. doi: 10.1097/00007632-199007000-00016. PMID: 2218718. 5. Data on File. 6. American Medical Electronics. (1986). PMA P850007: Physio-Stim Summary of Safety and Effectiveness Data. 7. OrthoLogic. (1999). PMA P910066: Spinalogic Bone Growth Stimulator Summary of Safety and Effectiveness.
References:
1. U.S. Food and Drug Administration. (2020). PMA P190030: FDA Summary of Safety and Effectiveness Data.
2. Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine. 1999;24(13):1349-1356.
3. Brighton CT, Wang W, Seldes R, Zhang G, Pollack SR. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am. 2001;83-A(10):1514-1523.
4. Mooney V. A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine. (Phila Pa 1976). 1990 Jul;15(7):708-12. doi: 10.1097/00007632-199007000-00016. PMID: 2218718.
5. Data on File.
6. American Medical Electronics. (1986). PMA P850007: Physio-Stim Summary of Safety and Effectiveness Data.
7. OrthoLogic. (1999). PMA P910066: Spinalogic Bone Growth Stimulator Summary of Safety and Effectiveness.





| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |


