CONTINUOUS WEARABILITY
Fits into your patient’s pocket — and their life

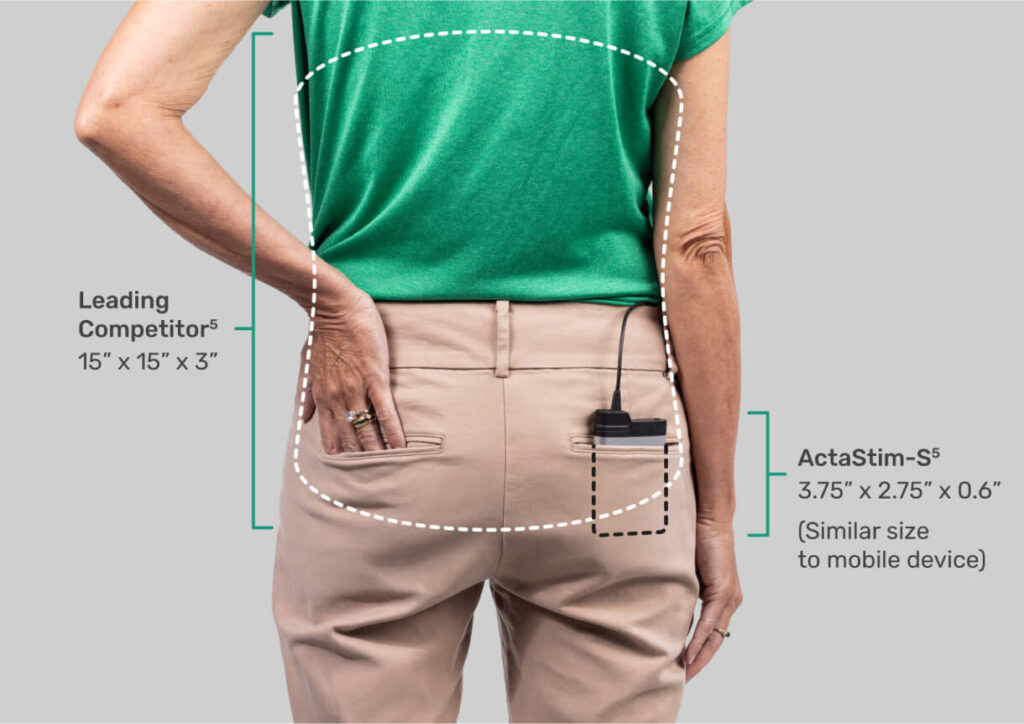
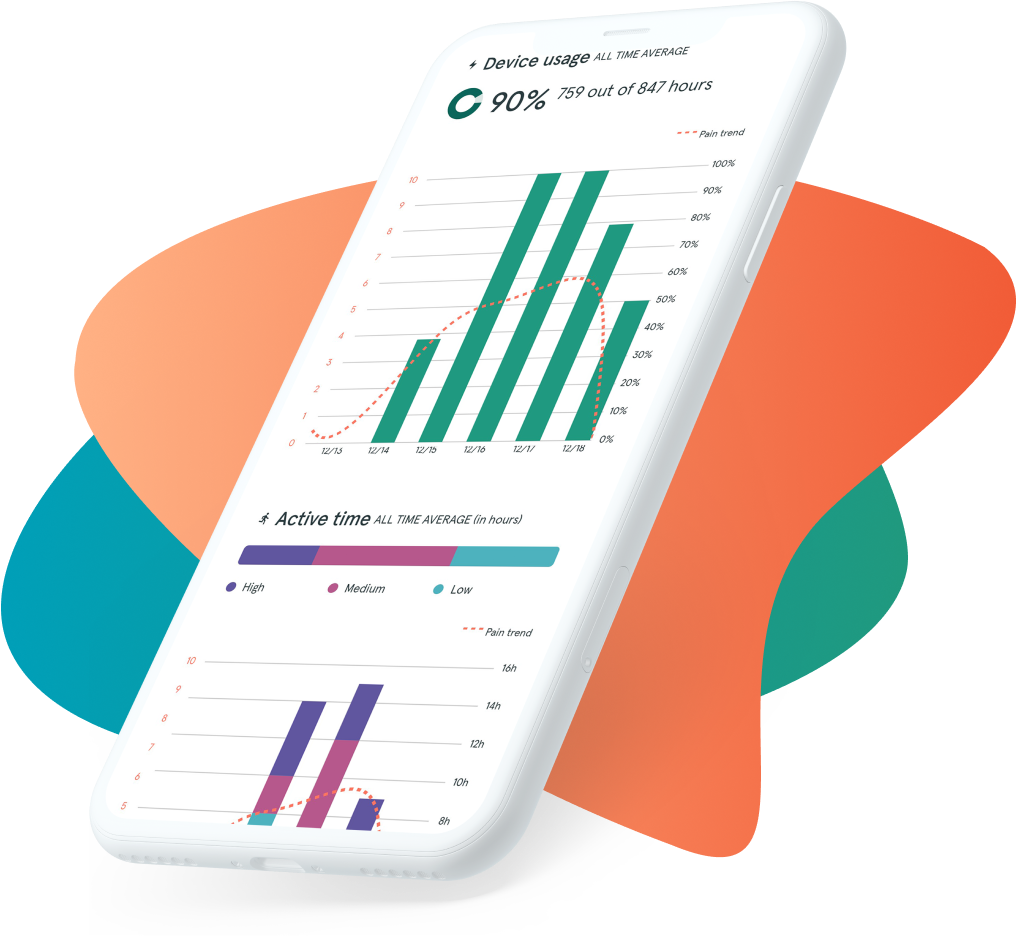
As the most compact BGS,5 the discreet and portable ActaStim-S makes it easier for patients to follow their recovery regimen, including:
Wear time — Patients can simply tuck it into a pocket, clip it on their belt, or set it on a nearby surface.
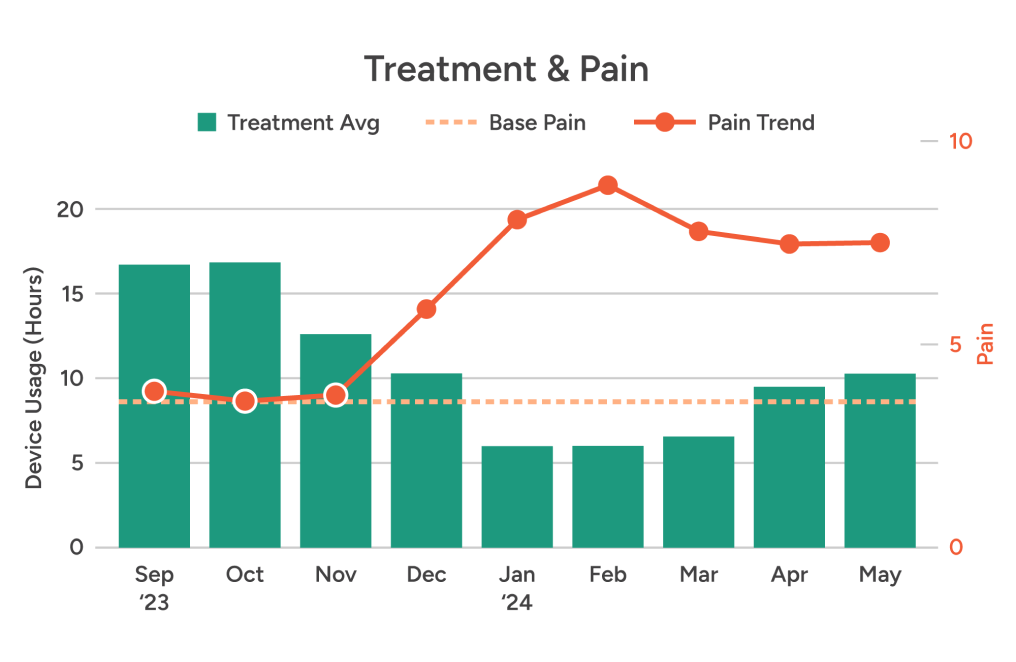
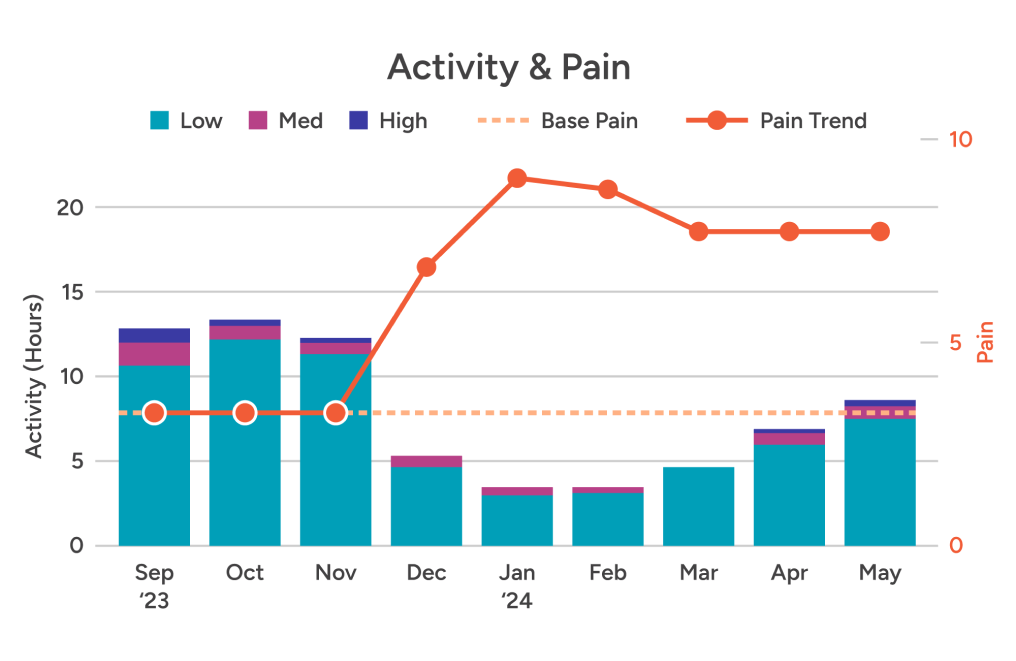
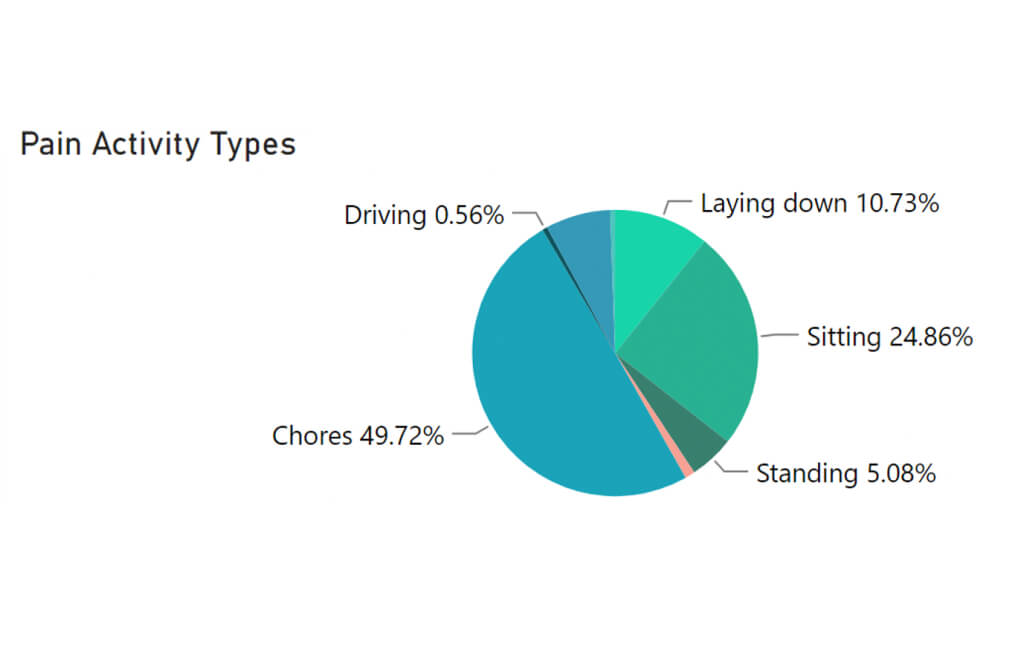
Physical activity — Unlike bulky, older devices, ActaStim-S doesn’t restrict movement, limit postural positions, or require patients to schedule their lives around using it.